画像をダウンロード 4n 2 rule explained 845355-4n+2 rule explained
Huckel Aromaticity And Frost Circles Organic Chemistry Help
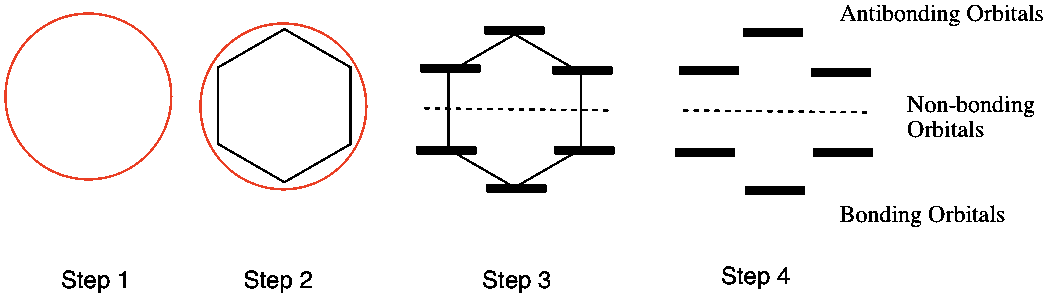
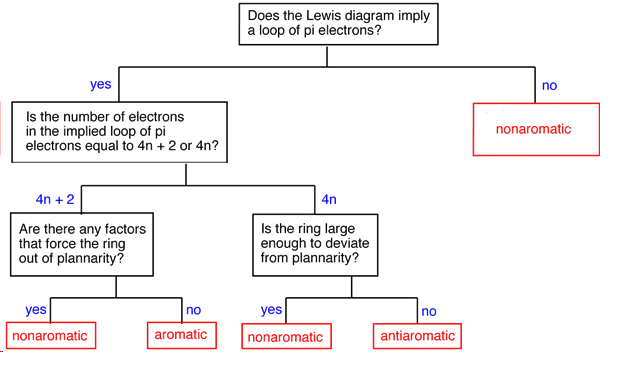
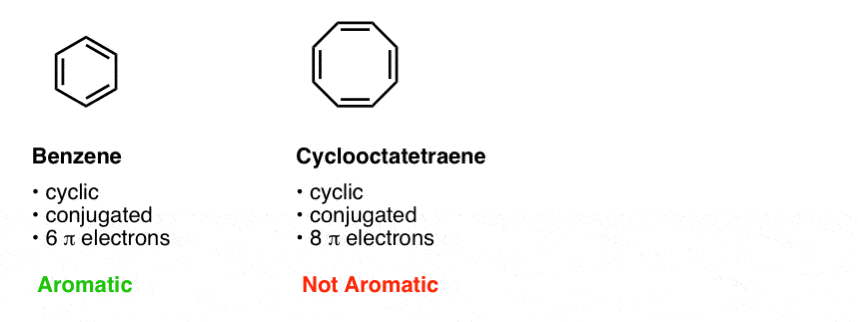
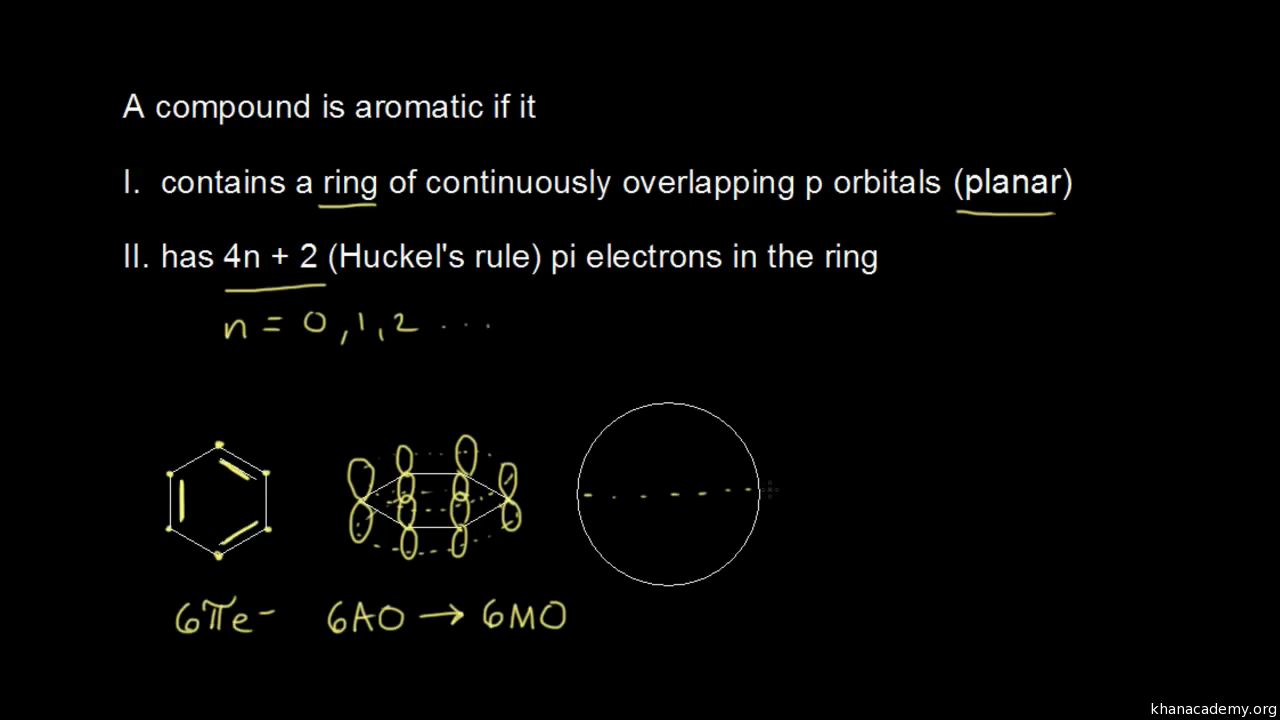
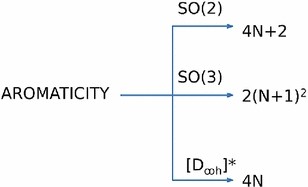
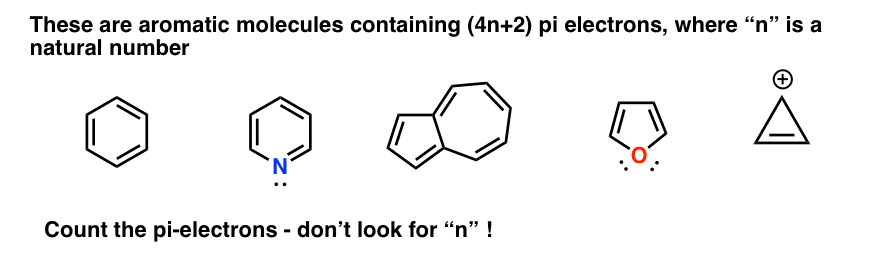
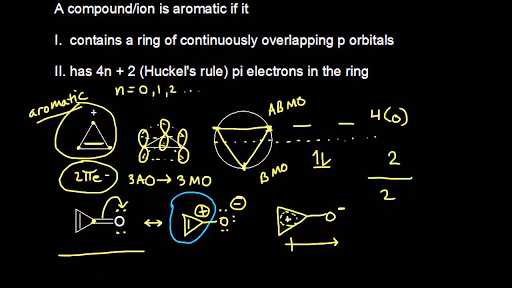
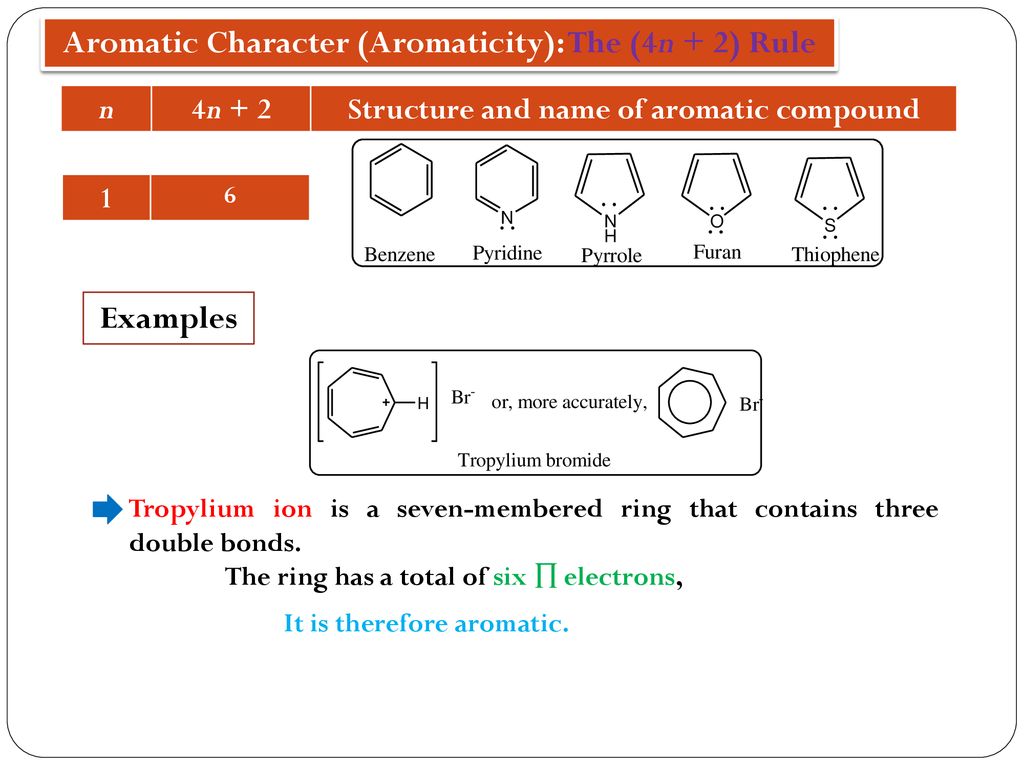
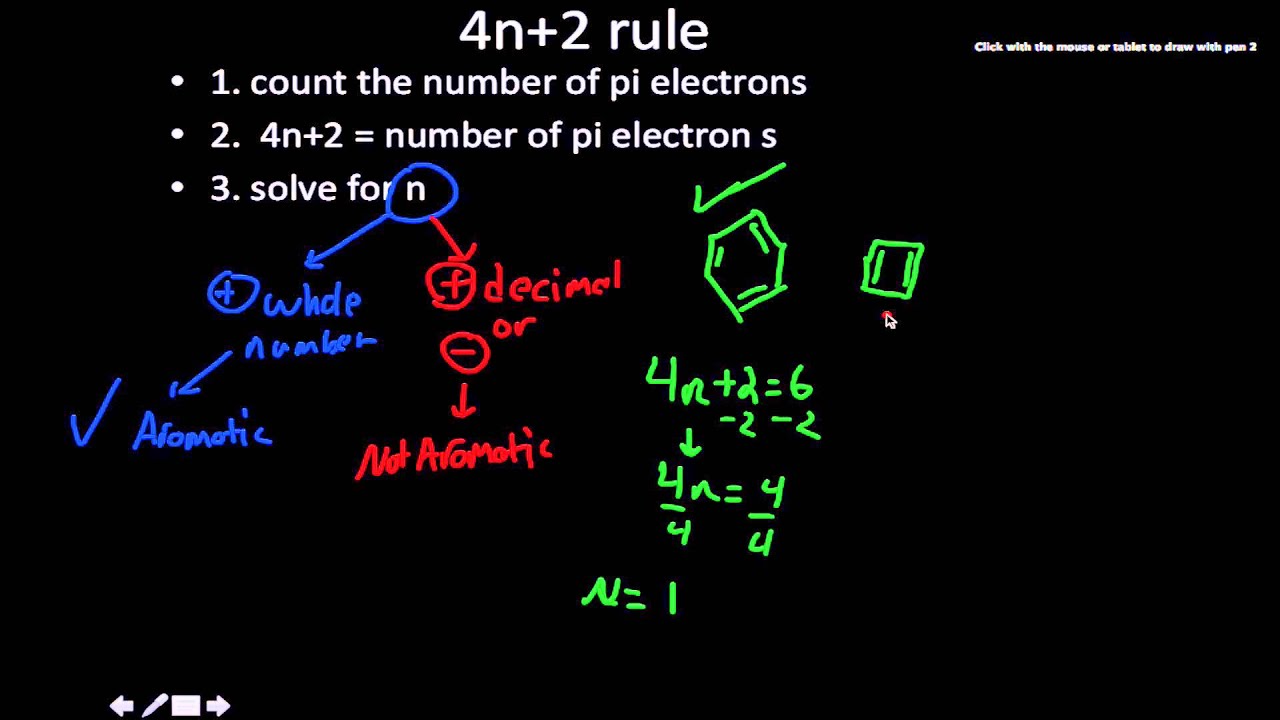
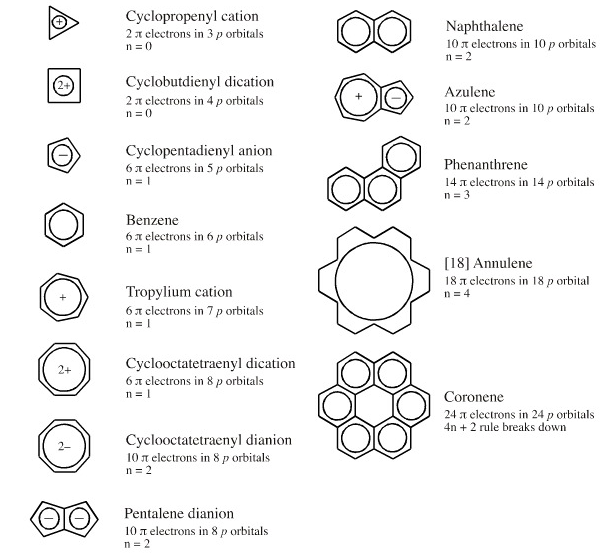
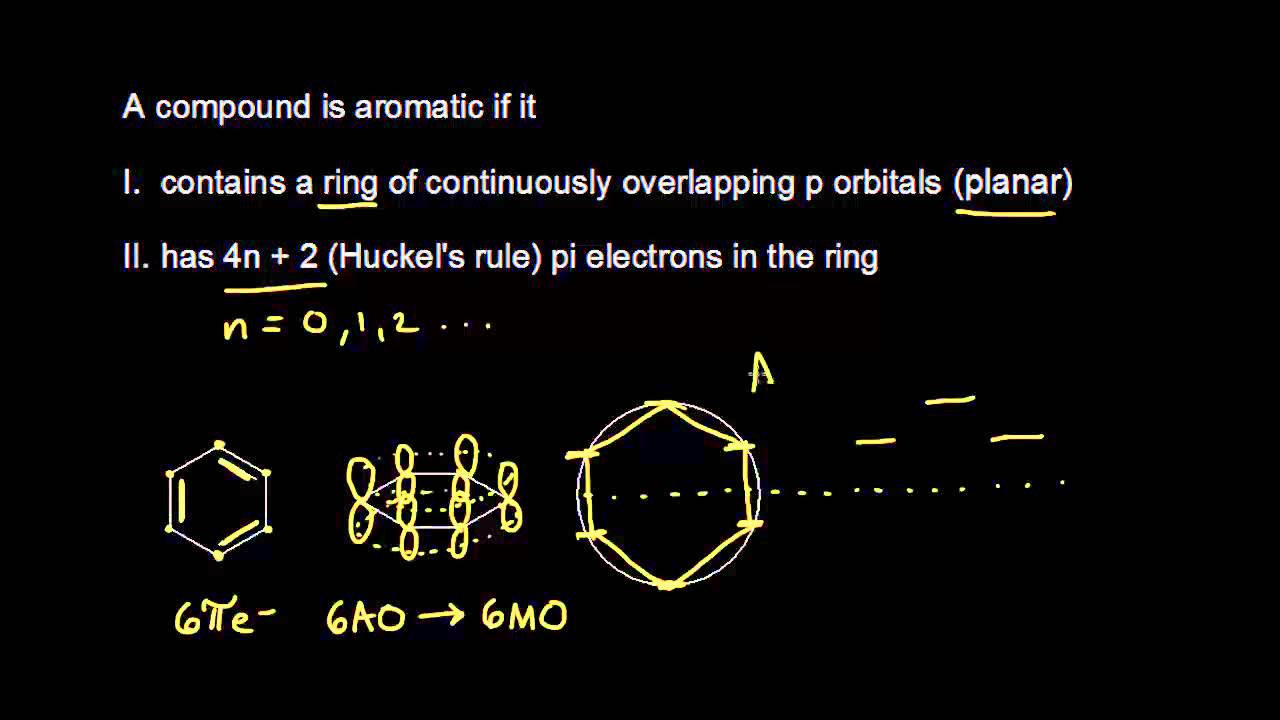
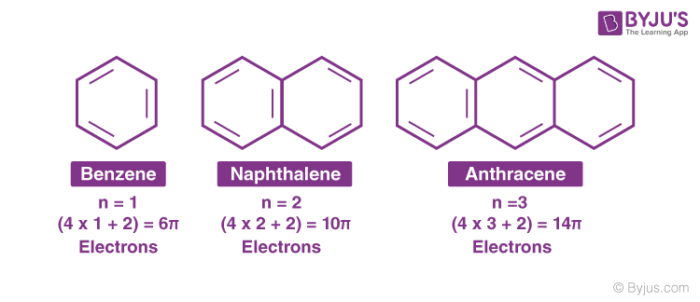
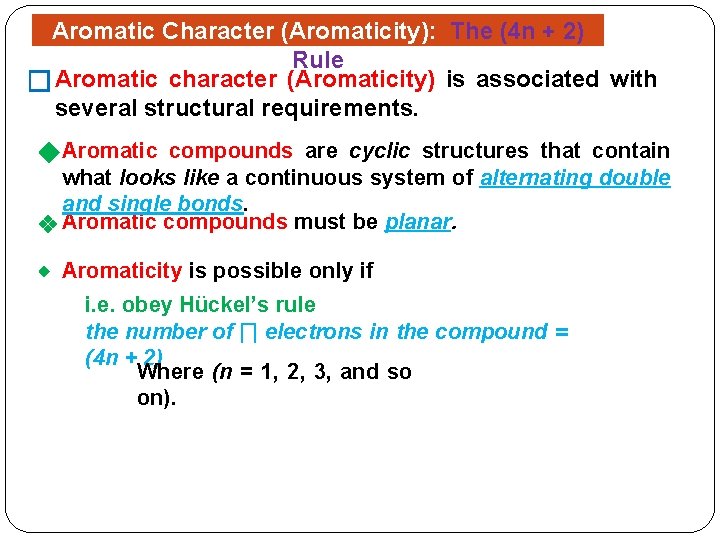
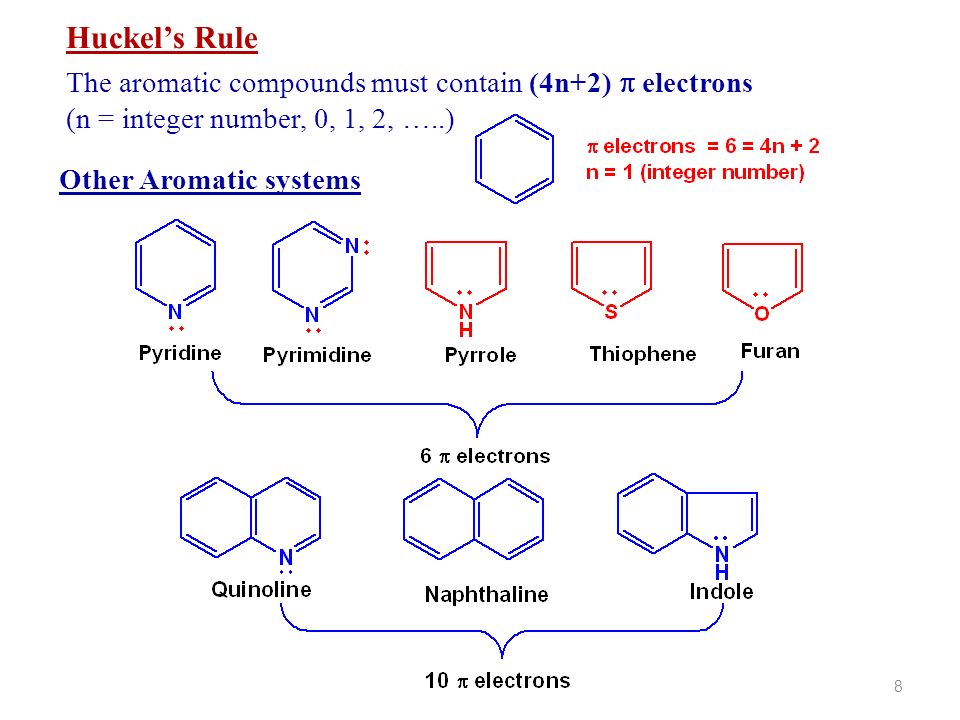
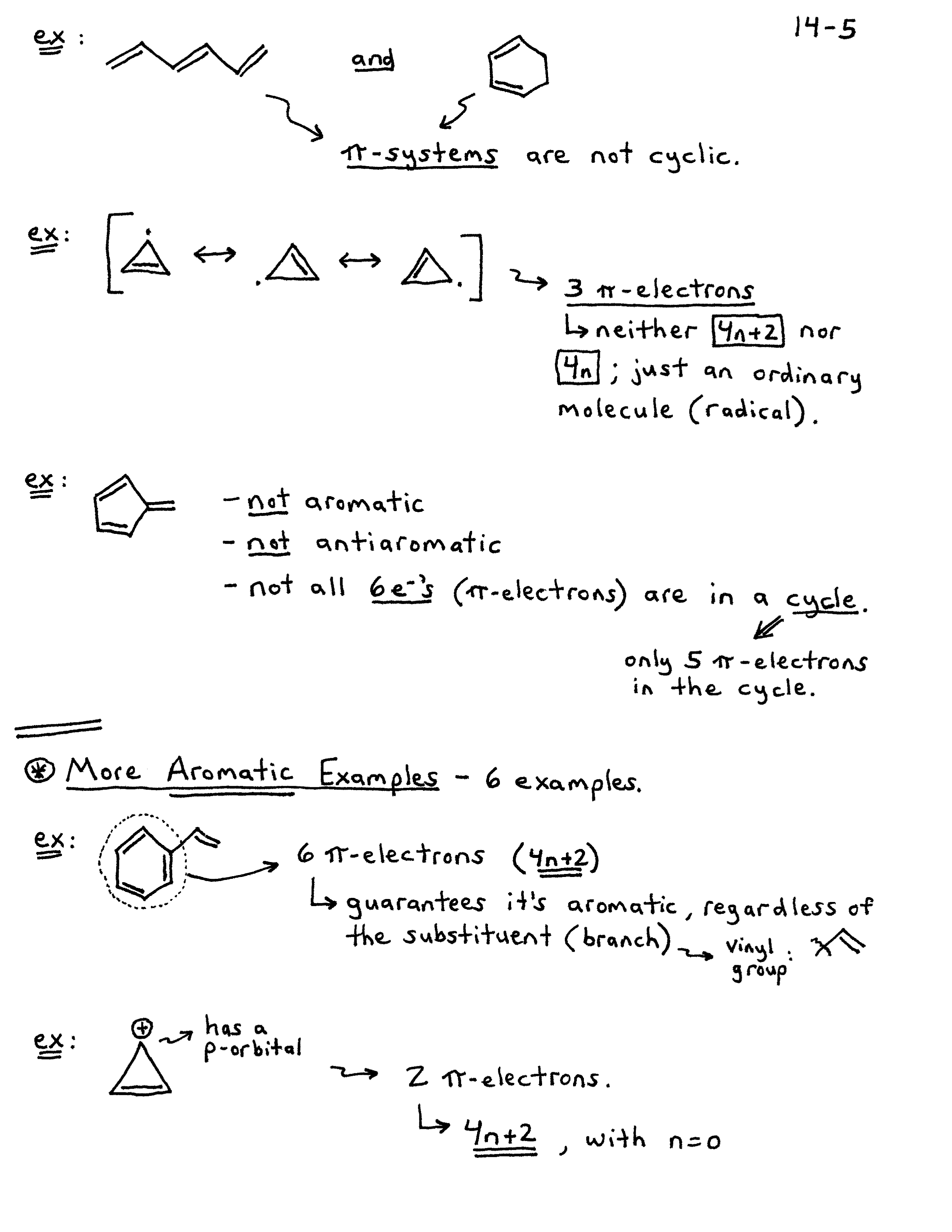
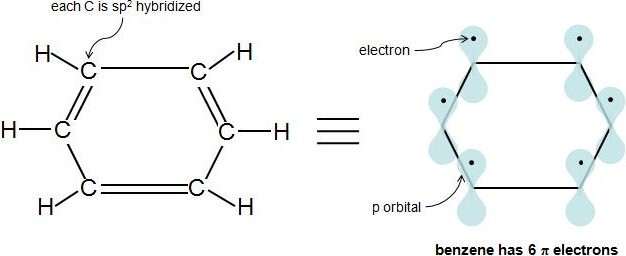
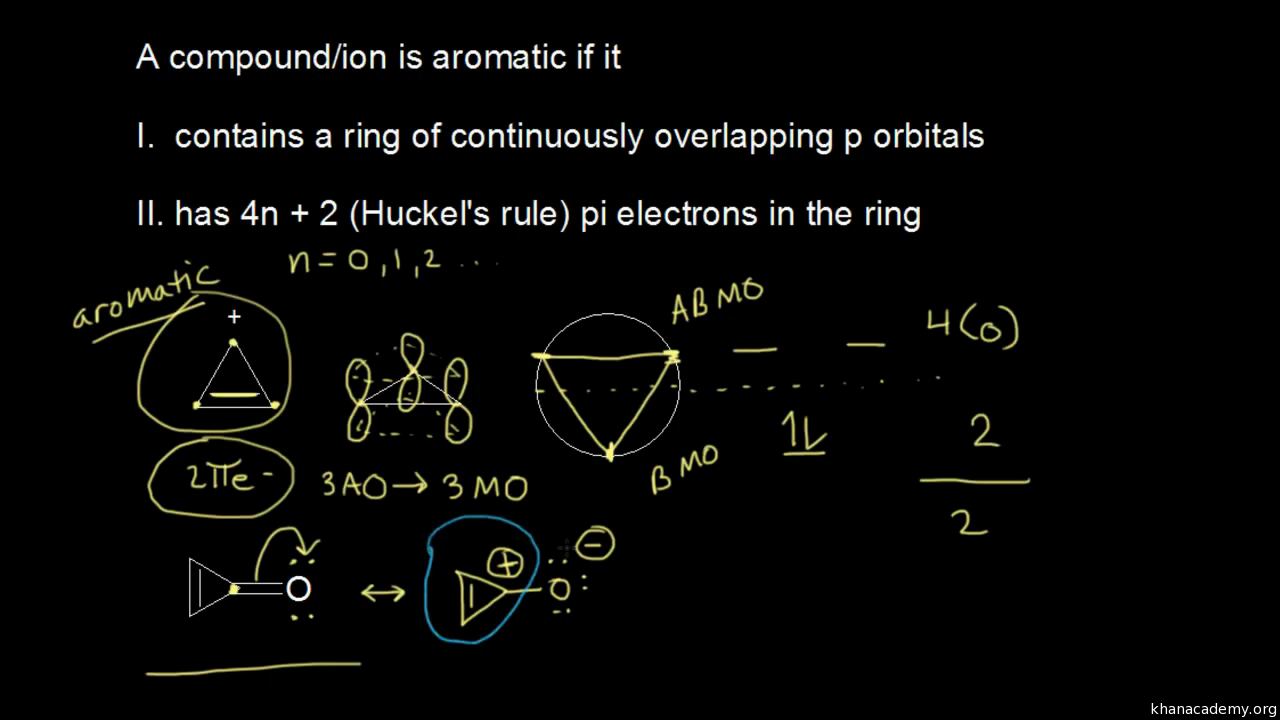
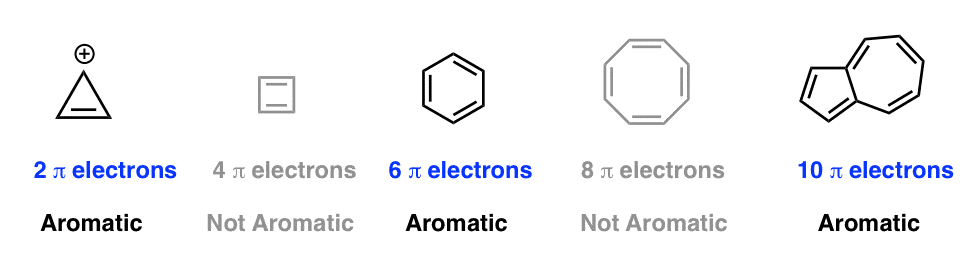
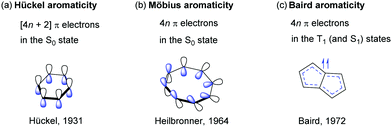
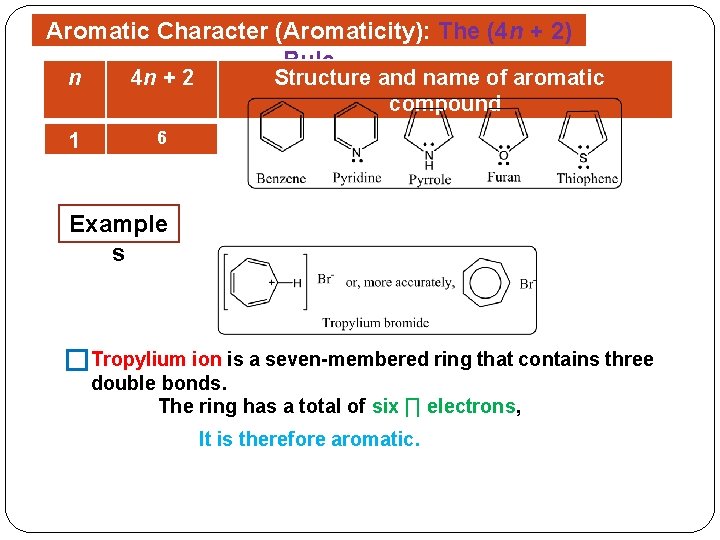
The Huckel aromaticity rules are Molecule is cyclic;In 1931, German chemist and physicist Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties His rule states that if a cyclic, planar molecule has 4n2 π electrons, it is considered aromatic This rule would come to be known as Hückel's Rule
4n+2 rule explained
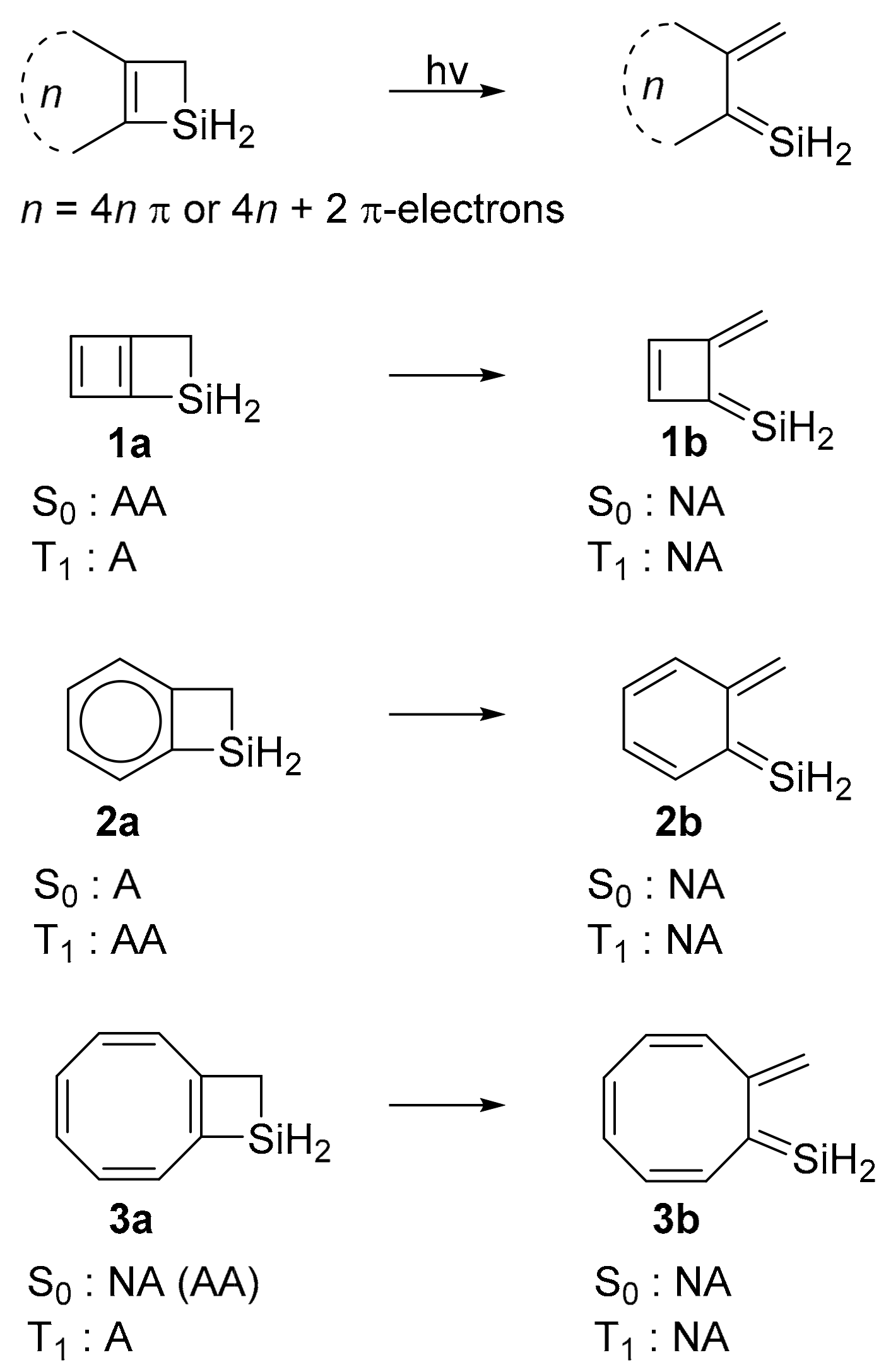
4n+2 rule explained-It follows the same 4n2 rule as for benzene, but this time counting the electrons involved in the reaction rather than just the πelectrons in the aromatic ring The advantage of this approach is that aromaticity is quite stable to perturbations to the symmetry (benzene rings in eg cyclophanes for example can be bent by up to 30° and still retain their aromaticity as evidenced by eg NMR)2 Introduction πConjugated compounds comprised of fused (4n2) and 4n electron cycles, such as biphenylene and Nphenylenes, are interesting for applications in organic and molecular electronics13 Yet, they are also of fundamental importance as their study sheds light on the limits of aromaticity and antiaromaticity, and ultimately on chemical bonding48 In their

Connecting And Combining Rules Of Aromaticity Towards A Unified Theory Of Aromaticity Sola 19 Wires Computational Molecular Science Wiley Online Library
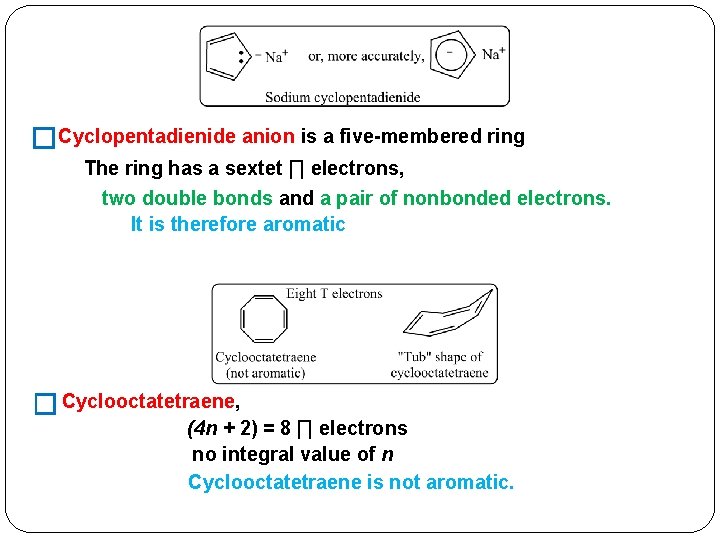
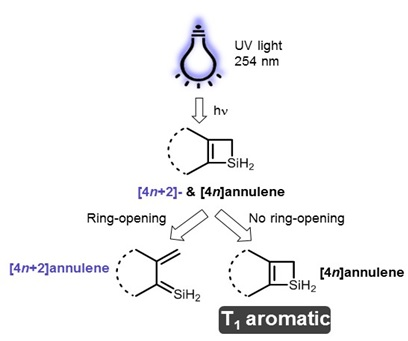
Furthermore, the 4n2 rule as indicator of aromatic stabilization should only be used in conjunction with the ring size;4n2 π electrons, photochemically antiaromatic and less stable Rules 2 and 4 were added in the 1960s, as the quantum mechanical understanding of photochemically excited states (open shell systems with two molecular orbitals each singly occupied) developed n is just any natural number which is used to satisfy the 4n 2 rule If that number becomes equal 4n 2 for any value of n then that compound is aromatic(or in other words if the number of pi electrons come in the series – 2, 6, 10, 14,
The "4n 2 rule" is derived analytically at the level of the simple Hückel theory for neutral evenmembered chains, their double ions, as well as cations and anions of the oddmembered chains, by determining the first order energetic effect of the ring closureThe topological background of the "4n 2 rule" is also briefly discussedThe nature of the occupied π orbitals must always be examined Discover the this video explains the experimental results of electrocyclic reactions involving 4n and (4n 2) pi systems with the help of woodward hoffmann rule this video dr norris reviews the woodward hoffmann rules for electrocyclic reactions and does some example problems the following videos contain the explanation for in this video the experimental results for (4n 2)pi
4n+2 rule explainedのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
 |  | |
 | .png?revision=1) | |
 |  |  |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  | |
 |  | |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 |  |  |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
.jpg?revision=1&size=bestfit&width=317&height=254) | 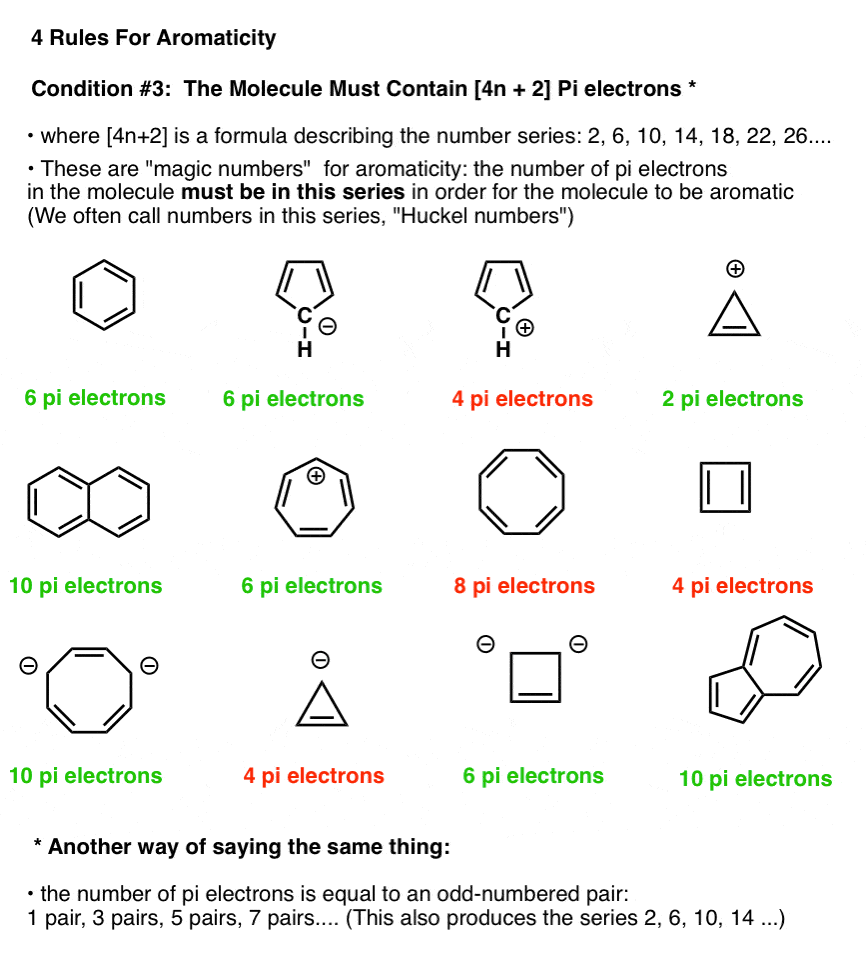 |  |
 |  |  |
 |  |  |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 |  |  |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  | |
 |  | |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 | .jpg?revision=1) | |
 |  | |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 | ||
 |  |  |
 |  | |
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 | ||
「4n+2 rule explained」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
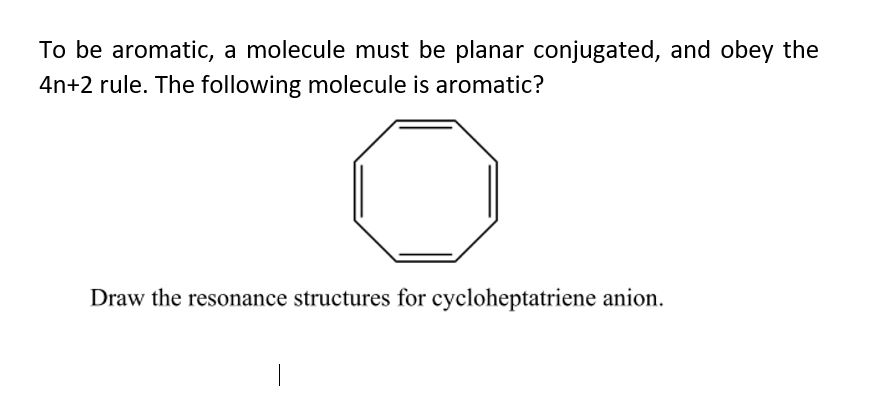 |  |
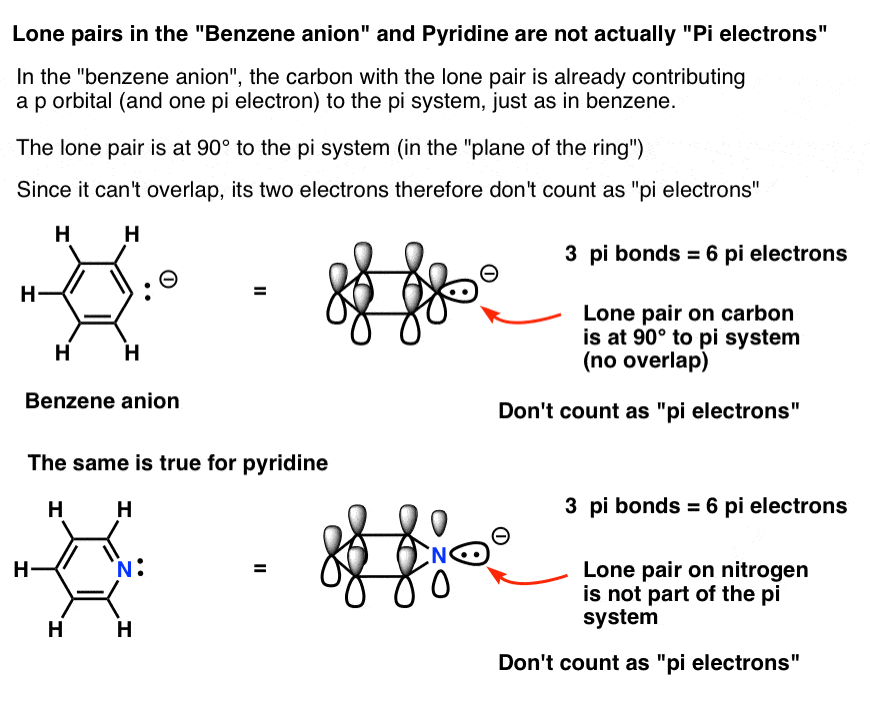
Like ak47 said sometimes you dont count the lone pairs other times you do So you take all the pi electrons and lone pairs (when applicable to be included) and set them equal to 4n2 electrons For example the number 10 is a huckel number because 4n2=10 n=2 and as long as n=0,1,2 (an integer) it passes the test UpvoteHilckel\u27s 4n 2 rule can be extended to interactions between the cationic center and pseudon orbitals of CH3, CH2 and CH groups respectively Thus, in analogy to hyperconjugation one can envisage homohyperconjugation as the probable cause of normal ydeuterium isotope effects
Incoming Term: 4n+2 rule explained,
コメント
コメントを投稿